The clean room, a contamination-controlled environment in which the level of air quality is extremely high, is used to carry out certain production activities involving specific requirements for the absence of impurities in the pharmaceutical and medical sectors. In this case, both the structure and the technology concerned must meet certain criteria to ensure the sterility of the environment. Rollon has developed several solutions for linear movement in clean rooms, including a seventh axis for robots.
Some industries require a sterile and aseptic production environment in order not to affect product quality. These include the medical and pharmaceutical industry, where there are very stringent regulations on product development, manufacturing and packaging, as they directly impact human life and health, and the safety of the operators themselves.
To meet the required standards, the production environment must therefore be a clean room: a room with a controlled atmosphere that allows for extremely pure air, in other words with a very low content of suspended microparticles.
Achieving this requires the constant filtration of air through HEPA filters; continuous monitoring of various parameters such as temperature, humidity, and pressure, the values of which vary depending on the function of the clean room; and various other measures to ensure that neither human presence nor the process and technology used for it alters the conditions of the clean room.
The classes of air cleanliness in clean rooms.
Production in clean rooms is regulated by UNI EN ISO 14644-1. This standard, which is part of the broader framework of the UNI EN ISO 14644 standard, defines air cleanliness classes and their expected particle contents by counting the airborne microparticles in a cubic meter of treated air.
The classes defined by the standard are 9 (ISO 1-9), where the first level indicates the absolute most controlled environment (10 particles of 0.1 µm, 2 particles of 0.2 µm) while the ninth indicates the least controlled environment (35200000 particles of 0.5 µm, 8320000 particles of 1 µm, 293000 particles of 5 µm), after which are the Non-Classified (N.C) environments.
Rollon’s response to the needs of the pharmaceutical and medical industry
The management of contamination-controlled environments involves more than just the filtration of air, adherence to certain environmental parameters and the control of contamination due to personnel: the structure and technology in the clean room must also be subject to strict rules. In fact, one of the main sources of contamination in the clean room is precisely the production process and the machinery used to carry it out.
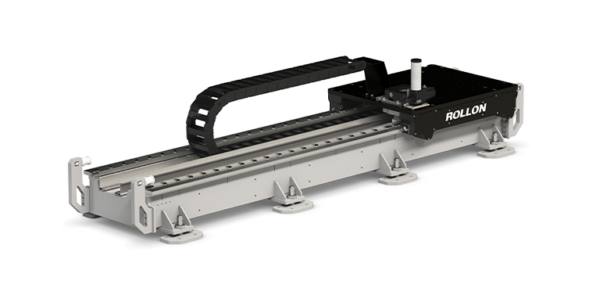
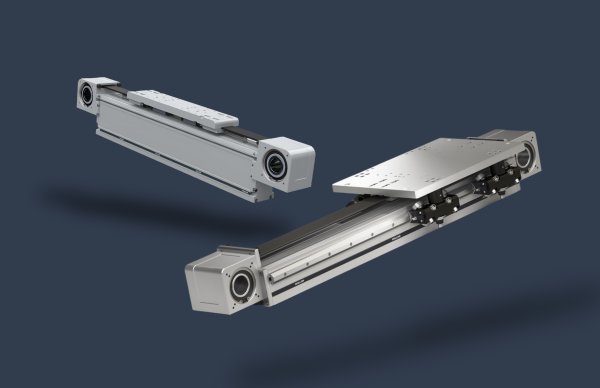
Since linear motion technology is a key asset for the production process in many areas of the pharmaceutical and medical industries, Rollon has developed the Clean Room System, a linear module designed precisely to ensure the lowest possible level of contamination of electromechanical elements installed on machinery in the clean room.
Clean Room System linear modules feature a belt drive and rails with recirculating ball bearings for movement. They are completely enclosed in a special protective cover and are suitable for use with a special vacuum system that provides a vacuum of 0.8 bar. This feature prevents the potential dispersion of particles into the environment where the system is installed, helping to ensure compliance with clean room requirements and cleanliness classes.
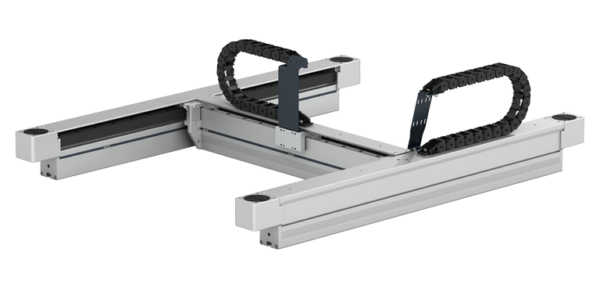
The seventh axis for clean rooms
With the Clean Room System actuator, Rollon has, together with its partners and customers, developed over the years multiple applications in disparate sectors: from semiconductors to pharmaceutical and medical, for example, for the production of syringes, vials and contact lenses. Backed by this experience, Rollon is now able to develop a seventh axis for the handling of robots based precisely on the Clean Room System family and therefore suitable for use in clean rooms.
The robot transfer unit for clean rooms combines the advantages of the Clean Room System range with those of robot handling systems, namely:
- extension of the range of the robot;
- durability over time;
- ability to handle small and medium-sized components and robots with different load capacities and sizes;
- strokes up to 10 meters;
- possibility of floor, wall and ceiling installation;
- certification up to class 1 according to UNI EN ISO 14644-1 depending on the characteristics of the application and different sizes of actuator.
The seventh axis is driven by a pair of belts in parallel, and each of its two component axes is driven by rails and recirculating ball bearings. In both the single axis and the seventh axis, special strips seal the slots of the profiles so as to ensure the highest level of cleanliness. In addition, the robot transfer unit features a robot interface and housings for proximity sensors.
Finally, through collaboration with various partners, Rollon is also able to supply gearboxes designed for use in clean rooms.